Stem cell–based treatment controls blood sugar in people with type 1 diabetes
An innovative stem cell–based treatment for type 1 diabetes can meaningfully regulate blood glucose levels and reduce dependence on daily insulin injections, according to new clinical trial results from the University of British Columbia and Vancouver Coastal Health.
The findings, published in Nature Biotechnology, arise from a multicentre clinical trial for an experimental cell therapy developed by US biotechnology company ViaCyte (acquired by Vertex Pharmaceuticals) that is being clinically tested in Canada.
The therapy aims to replace the insulin-producing beta cells that people with type 1 diabetes lack. Dubbed VC-02, the small medical implant contains millions of lab-grown pancreatic islet cells, including beta cells, that originate from a line of pluripotent stem cells. The devices are approximately the size of a Band-Aid and no thicker than a credit card. They are implanted just beneath a patient’s skin, where it is hoped they will provide a steady, long-term regulated supply of self-sustaining insulin. Co-author Dr Timothy Kieffer, a professor in the Departments of Surgery and Cellular and Physiological Sciences at UBC, and past chief scientific officer of ViaCyte, explains that pancreatic islet cells, grown from stem cells, are packaged into the device to recreate the blood sugar–regulating functions of a healthy pancreas.
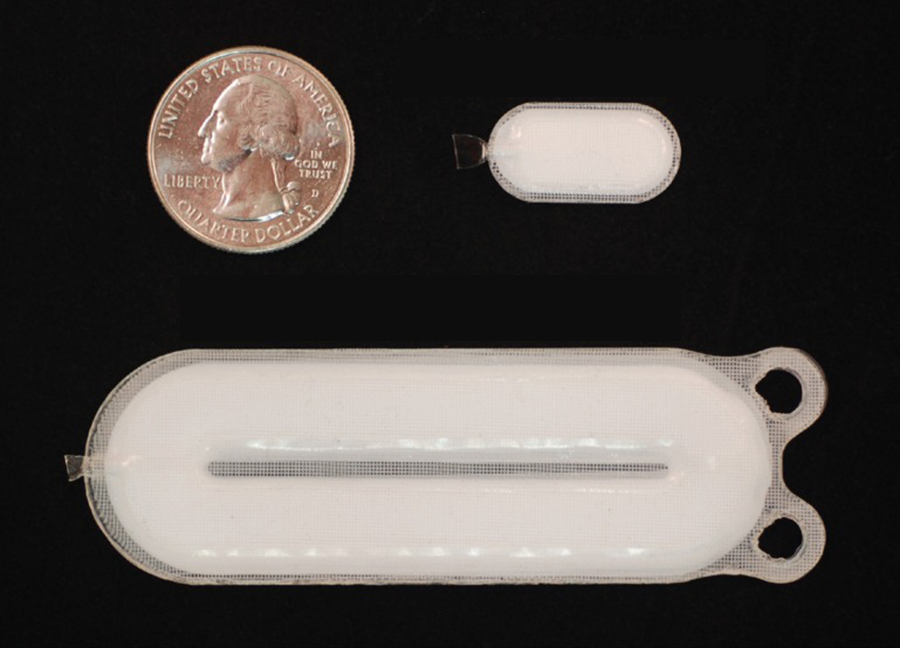
The dose-delivering unit (bottom) is about 7 cm long and is implanted along with a smaller sentinel device (top right). They are shown in comparison to a US quarter. (Credit: ViaCyte)
The clinical trial was conducted at Vancouver General Hospital, with additional sites in Belgium and the US. Ten participants, each of whom had no detectable insulin production at the start of the study, underwent surgery to receive up to 10 device implants each.
Six months later, three participants showed significant markers of insulin production and maintained those levels throughout the remainder of the year-long study. These participants spent more time in an optimal blood glucose range and reduced their intake of externally administered insulin. One participant increased their time spent in the target blood glucose range from 55% to 85%, and a showed 44% reduction in their daily insulin administration.
Dr David Thompson, principal investigator at the Vancouver trial site, clinical professor of endocrinology at UBC, and director of the Vancouver General Hospital Diabetes Centre, identifies this treatment as a significant step toward a functional cure for type 1 diabetes. With further refinement of this approach, researchers expect a therapy that can eliminate the need for daily insulin injections.
In a separate ongoing trial, the team is investigating whether a version of the device containing cells that have been genetically engineered to evade the immune system, using CRISPR gene-editing technology, could eliminate the need for participants to take immunosuppressant drugs alongside the treatment.
These trials continue to recruit participants. If you are interested in participating and want to learn more, contact study coordinator Barbara Allan (barbara.allan@vch.ca).
hidden
 |
| This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. |
