Epidemiology of Lyme disease and pitfalls in diagnostics: What practitioners need to know
ABSTRACT: Lyme disease is a tick-transmitted infection caused by Borrelia burgdorferi. Its prevalence is quite low in British Columbia compared with eastern North America. BC has a long history of studying Lyme disease. Extensive fieldwork and passive surveillance data clearly showed the low prevalence of this spirochete in the vector (ticks) and the predominant host (deer mice). The number of annual confirmed Lyme disease cases in BC is low, consistent with field epidemiological data. Lyme disease diagnosis is usually straightforward but can be more complex. In early Lyme disease, diagnosis is mainly clinical in patients with typical findings, a history of exposure in a Lyme-endemic region, and a history of having an attached engorged tick. For early disseminated and late Lyme disease, recommended public health laboratory tests using optimal blood samples are helpful. Recognition of erythema migrans following a tick bite is considered diagnostic; however, correct recognition of erythema migrans rash can be challenging in low-endemic areas such as BC. For serologic testing when needed, the BC Centre for Disease Control Public Health Laboratory adheres to the universally accepted Lyme disease testing guidelines, and recently introduced the newly recommended modified two-tiered test algorithm for diagnosis. There are other commercial alternative tests; however, the quality of those tests is often questionable, and their use is not recommended.
Accurate clinical diagnosis of Lyme disease based on the most common finding, erythema migrans, can be challenging, but in later stages, is aided by established serologic testing.
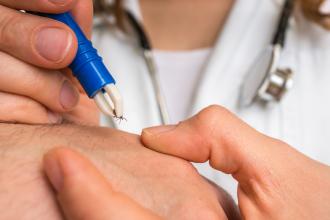
Lyme disease is a tick-transmitted infection caused by Borrelia burgdorferi. It is transmitted by Ixodes ticks, colloquially called deer ticks. In British Columbia, the disease is transmitted predominantly by I. pacificus; in central and eastern Canada, it is transmitted by I. scapularis. Ticks have a 2-year life cycle and three stages; the second (nymph) and third (adult) stages transmit Lyme disease. Ticks are usually encountered in grassy or wooded areas. They typically must be on the host at least 24 to 72 hours to transmit infection,[1-3] at which stage they are engorged [Figure 1]. Often, but not always, the engorged tick is seen.
There are three stages of Lyme disease: early localized, early disseminated, and late Lyme. Despite the diversity of potential manifestations, there are usually three common and typical presentations and two less typical common presentations. The first, erythema migrans, is seen 3 to 30 days after infection [Figure 2]. If not treated, early disseminated manifestations arise weeks to months after the bite, and present as a disseminated erythema migrans rash, and/or a radiculopathy, most commonly Bell palsy. Rare second stage presentations are carditis (usually heart block), meningitis, or encephalitis. There may also be polyarthralgias or a localized arthritis at this stage, but oligoarthritis, the third common presentation, is more typically seen in a third stage. Neurologic disease is also sometimes diagnosed at a later stage. Any stage, but particularly the first and second stages, may have nonspecific systemic symptoms. In reported cases of Lyme disease, patients experienced multiple signs: 70% had erythema migrans, 30% had arthritis, 9% had Bell palsy, 4% had another radiculoneuropathy, 2% had meningitis or encephalitis, and 1% had carditis.[4] Despite being promoted by some groups, there is no compelling evidence of serious posttreatment Lyme disease or of a host of nonspecific or other diseases caused by Lyme disease.
In dealing with the consideration of Lyme disease, clinicians encounter two categories of patients. We discuss primarily the first—those with Lyme disease or probable Lyme disease. However, and particularly in BC, where Lyme disease occurs infrequently, physicians often encounter patients who think or believe they have Lyme disease in the absence of compelling evidence. They usually do not have a clear exposure history or a documented rash consistent with Lyme disease, or do not have any of the other typical objective manifestations of Lyme disease. Additionally, these patients do not have positive laboratory tests done in an accredited lab but do usually have myriad highly distressing and life-altering symptoms, have often had courses of antimicrobials and other medications that may result in a transient “response” but do not cure them, and often have a “positive” test using one of the tests that are not recommended. These individuals deserve a thorough assessment for conditions other than Lyme disease. This is discussed near the end of this article.
Epidemiology and distribution of ticks and Lyme disease in BC
Historically, BC was well ahead of any other province in Canada in terms of studying ticks and tick-borne diseases. Tick research began in the early 1950s at the Canada Agriculture Research Station in Kamloops and played a key role in tick surveillance and determining the distribution of vector-borne and zoonotic diseases. This is reflected in a map created by JD Gregson, which was printed in the Proceedings of the Eighteenth Annual Meeting of the International Northwest Conference on Diseases Communicable to Man [Figure 3]. Gregson also wrote a monograph on ticks; he found a high diversity of ticks in Canada, and particularly emphasized BC ticks.[5] Recently, Morshed and colleagues collated 17 years of passive tick surveillance data from 2002 to 2018 and analyzed them to determine the occurrence of tick species and the prevalence of Borrelia spp. in ticks in BC. The authors reported 29 different tick species distributed throughout BC.[6] The predominant species are Ixodes pacificus, Dermacentor andersoni, and I. angustus. I. pacificus is more concentrated in the Lower Mainland and on Vancouver Island, D. andersoni (not a competent vector for B. burgdorferi) is more common in the Interior, and I. angustus is found throughout BC but low in numbers compared with I. pacificus and D. andersoni. Both I. pacificus and I. angustus were found to carry B. burgdorferi and are the principal vectors for transmitting Lyme disease in BC. The number of human tick submissions increased significantly (P < 0.001) between 2013 and 2018, but only 31 (0.28%) of 11 155 B. burgdorferi–carrying ticks were positive when tested either by culture or by polymerase chain reaction test.[6] Later, we added 2019 data [Figure 4] and found similar patterns. It is impossible to determine what percentage of tick-bitten patients will develop Lyme disease in a low-endemic area such as BC.
I. pacificus ticks are distributed across southern BC, predominately in the Greater Vancouver area and on Vancouver Island, but they have been detected as far north as Smithers (54°80’N, 127°20’ W) based on active surveillance.[7,8] The deer mouse (Peromyscus maniculatus), the major mammalian reservoir for B. burgdorferi in BC, has a widespread distribution in BC and acts as a common host for larval and nymphal I. pacificus ticks. To determine the percentage of tick positivity for B. burgdorferi, 3500 deer mice were tested by culture: 30 (0.86%) were positive. In addition, 164 mice were tested for antibodies to B. burgdorferi: 6 (3.66%) were positive, demonstrating a low prevalence in this reservoir.[9] Farther inland, I. pacificus ticks are uncommon but may be dispersed by birds during spring migration.[10-12] The Rocky Mountain wood tick, D. andersoni, is common in southeastern BC. It is not a vector of Lyme disease[13] but can transmit rickettsial and bacterial pathogens.[14]
In BC, the first Lyme disease case was reported locally in 1988.[15] The first isolation of B. burgdorferi sensu stricto in BC was reported in an adult I. pacificus tick and an immature I. angustus tick in 1993.[16] Lyme disease is endemic in BC, but only a few proven cases occur every year, although a certain group believes that this is not a true reflection of Lyme disease cases in BC. As a result, an innovative capture–recapture methodology was used to determine the true number of cases of Lyme disease from 1997 to 2008. Conservative estimates placed the true number of Lyme disease cases in BC during this period at 142 (95% CI, 111–224), indicating up to 40% underreporting of this rare disease.[17] Morshed and colleagues analyzed BC case numbers from 2002 to 2018, along with available Canadian positivity rates.[6] The case numbers in BC remained quite low, ranging from 3 to 22 cases, except in 2016, when there were 40 cases. On average, half those cases were acquired during travel outside BC (data not shown). We added 2019 data and found a similar trend [Figure 5]. The Lyme disease case number in BC is much lower than that in eastern North America (e.g., Nova Scotia, Ontario, and Quebec [data not shown]), but is similar to that in the western US (Washington, Idaho, and California).[18]
Treatment
Evidence-based treatment of Lyme disease has been virtually the same for several decades, including in the 2020 guidelines by the Infectious Diseases Society of America, American Academy of Neurology, and American College of Rheumatology.[19] The mainstay of treatment is doxycycline 100 mg orally twice daily for 10 days, except for carditis or neurologic manifestations, in which case treatment is oral doxycycline or IV ceftriaxone for 14 to 21 days. Recommended treatment for arthritis is 28 days of oral doxycycline.
Alternative guidelines are widely promoted by some groups, such as the International Lyme and Associated Diseases Society. They involve longer and often more complicated regimens, including with other drugs. Although such regimens have been advocated for decades, no credible evidence has been provided to demonstrate their superiority, or in some cases any efficacy, and certainly no evidence has demonstrated that their benefits exceeds their risks.
Pitfalls in diagnosis
For most patients, Lyme disease is a clinical diagnosis in which the patient has typical manifestations, plus a history of exposure to a Lyme disease-endemic area, and a history of having an attached engorged tick. Lyme disease–carrying ticks, predominantly Ixodes spp., need to be attached for at least 24 to 72 hours.[1-3] In these clinical settings, treatment should be initiated as if the patient has Lyme disease; serologic support is not usually needed.
Ideally, early Lyme disease will be suspected based on the presence of a typical erythema migrans skin rash. In areas where Lyme disease is prevalent, such as Rhode Island, Pennsylvania, New Hampshire, Vermont, and Maine, where confirmed cases per 100 000 population ranged from 49.7 to 121.3 in 2019,[18] most people with such a rash will have Lyme disease. However, in low-prevalence areas such as BC (approximately 0.2 confirmed cases per 100 000 population), erythema migrans is often misdiagnosed. Typical erythema migrans arises 3 to 30 days after the bite, usually at the site of the bite, spreads slowly over days to weeks, is more than 5 cm in diameter—most typically 10 to 16 cm—and often has central clearing. In contrast, cases misdiagnosed often arise within 3 days (usually hours), are often itchy or indurated, do not reach 5 cm in diameter, and often resolve quickly. These are presumably an allergic reaction or a localized cellulitis. For example, Figure 7 shows a rash resembling erythema migrans in a patient from the West Coast, a low-prevalence area; the patient was seronegative and a diagnosis of Lyme disease was excluded. Even in Lyme-endemic areas, erythema migrans is frequently not diagnosed, and non–erythema migrans rashes are frequently called erythema migrans.[20,21]
Other clinical presentations such as Bell palsy or polyarthralgias are less specific for Lyme disease, but in the correct clinical context, they should prompt suspicion of Lyme disease, and in patients with exposure history in an endemic region, should prompt empiric treatment as if the patient has Lyme disease, which, as in all infections where empiric therapy is initiated, is not the same as diagnosing the disease. When Lyme disease is specifically diagnosed or seriously considered, a careful cardiac examination and potentially an ECG is indicated. Although deaths from Lyme disease are very rare, heart block is the most common cause. It responds well to standard antimicrobial Lyme disease treatment but may need transient pacing.
Laboratory diagnosis in BC
Serology or antibody testing is considered the test of choice for laboratory diagnosis of Lyme disease. In the early 1980s, immunofluorescence assay was used to screen Lyme disease, and all positive cases were further confirmed by western blot assay. A few years later, enzyme-linked immunosorbent assay or enzyme immunoassay replaced immunofluorescence assay because of notorious false positivity or nonspecific binding to the fluorescence dye. This enzyme-linked immunosorbent assay or enzyme immunoassay followed by western blot assay is evidence-based and was recommended at the Second National Conference on Serologic Diagnosis of Lyme Disease in October 1994[22] [Figure 6][23]. Later, this two-tiered approach was adopted universally in North America, Europe, and Asia.
The BC Centre for Disease Control (BCCDC) Public Health Laboratory also offered this standard two-tiered test from the early 1990s to May 2021. Since June 2021, the Public Health Laboratory has adhered to a new algorithm recommended by the US Centers for Disease Control and Prevention—the modified two-tiered test.[23,24] As an initial test, samples are screened by an enzyme-linked immunosorbent assay test using a polyvalent antigen. This is a very sensitive test, so it will detect antibodies to Lyme disease or to other infections that are similar to Lyme disease. If the test is “positive” or “indeterminant,” the sample is further tested by a specific and separate IgM and IgG enzyme immunoassay [Figure 6]. In addition, the BCCDC Public Health Laboratory uses western blots on suspected samples when the modified two-tiered test fails to provide discrete results.
Other diagnostic testing offered/available in BC
The BCCDC Public Health Laboratory has also developed a protocol for B. burgdorferi culture and a number of molecular tests based on polymerase chain reaction, and provides them as adjunct tests if necessary to rule out infection upon consultation with the test-ordering physician. Although specificity is quite good on these tests, sensitivity is less than 20%, even with the use of optimal samples, such as biopsy from the edge of erythema migrans rashes, synovial fluid from an inflamed joint, or cerebrospinal fluid from a neuro–Lyme suspected case. Positive results are rare, even in patients from highly endemic areas.
Alternative testing
Alternatively diagnosed Lyme disease—that is, Lyme disease supposedly diagnosed on the basis of nontraditional testing using tests that have variable sensitivity but poor specificity, misinterpretation of standard serology, or purely clinical grounds in patients with nonspecific symptoms—does not establish a diagnosis of Lyme disease. The accuracy and clinical usefulness of nontraditional tests, including urine antigen tests, immunofluorescent staining for cell wall–deficient forms of B. burgdorferi, lymphocyte transformation tests, CD57 natural killer cells, enzyme-linked immunosorbent spot (ELISpot) (IFN-γ secretion by T cells), and tests for B. burgdorferi DNA on inappropriate specimens such as blood and urine or in house-developed western blots using different interpretation criteria, have not been adequately validated.[25,26] Most of the public health or accredited laboratories do not recommend using these tests for laboratory diagnosis.[24] Inappropriateness of alternatively diagnosed Lyme disease is particularly relevant in low-prevalence areas such as BC. This was well demonstrated in a study of patients in BC who were labeled as having chronic Lyme disease without evidence provided by standard criteria.[27] When assessed by numerous standard and experimental approaches, none had any evidence of infection with B. burgdorferi, or indeed infection at all. Most patients fulfilled criteria for chronic fatigue/myalgic encephalitis and were clinically indistinguishable from a control group of patients diagnosed with chronic fatigue syndrome. Many of these patients are highly symptomatic and severely debilitated.[27] They deserve concerted diagnostic and management approaches but not long courses of putative regimens that are active against B. burgdorferi. The Association of Medical Microbiology and Infectious Disease Canada has strongly recommended that governments fund multidisciplinary clinics to provide comprehensive, compassionate, and evidence-based care for such individuals.
Assessment of patients in whom Lyme disease is unlikely
Such patients deserve a thorough assessment, both to carefully assess the likelihood of Lyme disease and to explore the cause(s) of their symptoms. Where the history, clinical findings, and sometimes laboratory tests suggest Lyme disease, these patients should be managed as discussed above, sometimes treating for Lyme disease as if they have Lyme disease—for example, those with an exposure history and new or recent typical erythema migrans rash without laboratory confirmation.
Where Lyme disease is not likely, alternative diagnoses must be considered based on a thorough history (e.g., travel, outdoor activities), examination, and consideration of other data. Most of these patients will have findings consistent with chronic fatigue/myalgic encephalitis syndrome, but there are numerous other possibilities—for example, sleep apnea, depression, substance use, multiple sclerosis, a rheumatologic condition, or cancer. The referral form for the Chronic Complex Diseases Program at BC Women’s Hospital and Health Centre lists many of the more common considerations.[28] Investigations are warranted to assess for common conditions, but as in all testing, they should be evidence-based, not a fishing expedition leading to significant risk of false-positive test results. Where Lyme disease is unlikely, no Lyme disease laboratory testing is warranted, and for most patients, no further testing for other pathogens (e.g., Epstein-Barr virus, Bartonella, Rickettsia) is warranted. Basic laboratory testing, as recommended for people with chronic fatigue, includes a complete blood count with differential chemistries (including glucose, electrolytes, calcium, renal, and hepatic function tests), thyroid-stimulating hormone, and creatine kinase (if muscle pain or weakness is present). Testing for HIV, syphilis, and hepatitis is warranted if results are not already known. Beyond that, investigations should be directed toward other considerations based on the patient’s history and examination.
Management of Lyme disease can be difficult and time-consuming. As noted above, the Association of Medical Microbiology and Infectious Disease Canada has strongly recommended that governments fund multidisciplinary clinics to provide comprehensive, compassionate, and evidence-based care for affected individuals. The Chronic Complex Diseases Program at BC Women’s is one such program.
Summary
Lyme disease is present in BC, but in low numbers. In the correct context, particularly in early Lyme disease, diagnosis is primarily clinical, without need for laboratory testing. However, in low-prevalence settings such as BC, accurate clinical diagnosis of the most common finding, erythema migrans, can be challenging. In later stages, diagnosis is aided by the use of established serologic testing, as performed by the BCCDC Public Health Laboratory. The sensitivity and specificity of these tests is well established. Alternative means of diagnosing putative Lyme disease should not be used.
Acknowledgments
We would like to thank the laboratory staff of the Zoonotic and Emerging Pathogens and Parasitology sections of the BCCDC Public Health Laboratory. We would also like to thank Drs Todd Hatchette and Robbin Lindsay for sharing one of the figures.
Competing interests
None declared.
This article has been peer reviewed.
 |
| This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. |
References
1. Falco RC, Fish D, Piesman J. Duration of tick bites in a Lyme disease-endemic area. Am J Epidemiol 1996;143:187-192.
2. Sood SK, Salzman MB, Johnson BJ, et al. Duration of tick attachment as a predictor of the risk of Lyme disease in an area in which Lyme disease is endemic. J Infect Dis 1997;175:996-999.
3. Wilhelmsson P, Lindblom P, Fryland L, et al. Ixodes ricinus ticks removed from humans in Northern Europe: Seasonal pattern of infestation, attachment sites and duration of feeding. Parasit Vectors 2013;6:362.
4. Schwartz AM, Hinckley AF, Mead PS, et al. Surveillance for Lyme disease—United States, 2008–2015. MMWR Surveill Summ 2017;66(No. SS-22):1-12.
5. Gregson JD. The Ixodoidea of Canada. Ottawa, ON: Queen’s Printer; 1956.
6. Morshed MG, Lee M-K, Boyd E, et al. Passive tick surveillance and detection of Borrelia species in ticks from British Columbia, Canada: 2002–2018. Vector Borne Zoonotic Dis 2021;21:490-497.
7. Mak S, Morshed M, Henry B. Ecological niche modeling of Lyme disease in British Columbia, Canada. J Med Entomol 2010;47:99-105.
8. Morshed MG, Lee M-K, Man S, et al. Surveillance for Borrelia burgdorferi in Ixodes ticks and small rodents in British Columbia. Vector Borne Zoonotic Dis 2015;15:701-705.
9. Henry B, Morshed M. Lyme disease in British Columbia: Are we really missing an epidemic? BCMJ 2011;53:224-229.
10. Morshed MG, Scott JD, Banerjee SN, et al. First isolation of Lyme disease spirochete, Borrelia burgdorferi, from blacklegged tick, Ixodes scapularis, removed from a bird in Nova Scotia, Canada. Can Commun Dis Rep 1999;25:153-155.
11. Morshed MG, Scott JD, Fernando K, et al. Migratory songbirds disperse ticks across Canada, and first isolation of the Lyme disease spirochete, Borrelia burgdorferi, from the avian tick, Ixodes auritulus. J Parasitol 2005;91:780-790.
12. Scott JD, Fernando K, Banerjee SN, et al. Birds disperse ixodid (Acari: Ixodidae) and Borrelia burgdorferi-infected ticks in Canada. J Med Entomol 2001;38:493-500.
13. Dolan MC, Maupin GO, Panella NA, et al. Vector competence of Ixodes scapularis, I. spinipalpis, and Dermacentor andersoni (Acari:Ixodidae) in transmitting Borrelia burgdorferi, the etiologic agent of Lyme disease. J Med Entomol 1997;34:128-135.
14. Dergousoff SJ, Gajadhar AJA, Chilton NB. Prevalence of Rickettsia species in Canadian populations of Dermacentor andersoni and D. variabilis. Appl Environ Microbiol 2009;75:1786-1789.
15. Burgess B. British Columbia. Lyme disease in horses. Can Vet J 1988;29:393-394.
16. Banerjee SN. Isolation of Borrelia burgdorferi in British Columbia. Can Commun Dis Rep 1993;19:204-205.
17. Henry B, Roth D, Reilly R, et al. How big is the Lyme problem? Using novel methods to estimate the true number of Lyme disease cases in British Columbia residents from 1997 to 2008. Vector Borne Zoonotic Dis 2011;11:863-868.
18. Centers for Disease Control and Prevention. Lyme disease data tables: Historical data. Reported cases of Lyme disease by state or locality, 2010-2019. Accessed 9 April 2022. www.cdc.gov/lyme/stats/tables.html.
19. Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis and treatment of Lyme disease. Clin Infect Dis 2021;72:e1-e48.
20. Lipsker D, Lieber-Mbomeyo A, Hedelin G. How accurate is a clinical diagnosis of erythema chronicum migrans? Prospective study comparing the diagnostic accuracy of general practitioners and dermatologists in an area where Lyme borreliosis is endemic. Arch Dermatol 2004;140:620-621.
21. Aucott JN, Crowder LA, Yedlin V, Kortte KB. 2012. Bull’s-eye and nontarget skin lesions of Lyme disease: An Internet survey of identification of erythema migrans. Dermatol Res Pract 2012;2012:451727.
22. Association of State and Territorial Public Health Laboratory Directors, US Centers for Disease Control and Prevention, and Michigan Department of Health. Recommendation. In: Proceeding of the second national conference on serologic diagnosis of Lyme disease. Washington, DC; 1994. pp. 1-11.
23. Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep 2019;68:703.
24. Hatchette T, Lindsay R. Modified two-tiered testing algorithm for Lyme disease serology: The Canadian context. Can Commun Dis Rep 2020;46:125-131.
25. US Centers for Disease Control and Prevention. Notice to readers: Caution regarding testing for Lyme disease. MMWR Morb Mortal Wkly Rep 2005;54:125.
26. Fallon BA, Pavlicova M, Coffino SW, Brenner C. A comparison of Lyme disease serologic test results from 4 laboratories in patients with persistent symptoms after antibiotic treatment. Clin Infect Dis 2014;59:1705-1710.
27. Patrick DM, Miller RR, Gardy JL, et al. Lyme disease diagnosed by alternative methods: A phenotype similar to that of chronic fatigue syndrome. Clin Infect Dis 2015;61:1084-1091.
28. BC Women’s Hospital and Health Centre, Complex Chronic Diseases Program. Symptoms attributed to chronic Lyme-like disease. Accessed 9 April 2022. www.bcwomens.ca/health-info/living-with-illness/symptoms-attributed-to-chronic-lyme-disease.
Dr Morshed is a program head and clinical microbiologist at the British Columbia Centre for Disease Control Public Health Laboratory (BCCDC PHL), and a clinical professor in the Department of Pathology and Laboratory Medicine, University of British Columbia. Mr Lee is technical coordinator at BCCDC PHL. Dr Bowie is a professor in the Division of Infectious Diseases, Department of Medicine, UBC.